1. Acceleration Initiative for Deep-Tech startups in Devices, Diagnostics and Digital Health – AID - A 16-week cohort based program
We help MedTech and Life Sciences innovations move faster from lab to market. Through collaboration and access to the right networks, we make it easier for new healthcare technologies to reach the people who need them most.


2. Mission 10X
A 6-month joint scaleup program for early-stage research connected startups. The program offered to startups will facilitate market strategy, corporate market reviews, customer connects and funding opportunities. The collaborative initiative will support the startup ecosystem by working with research connected startups from several incubators and accelerators within Telangana and outside.
3. International Engagements
Are you a MedTech Startup exploring to enter the Indian market and need guidance on understanding Market Research & Validation, Regulatory Compliance and Manufacturing in India?
Who is this program for?
- Startups from across the globe looking to enter Indian market are invited to apply for the scaleup support.
- RICH offers three streams, each designed to cater to international startups at different points in their business journey.
Market Research & Validation
Opportunity for introductions to public and private healthcare stakeholders for primary understanding of the market
Regulatory Compliance
Need assessment And Awareness and introductions for undertaking Regulatory Processes in India
Manufacturing
Need assessment And introductions for undertaking manufacturing in India
4. Corporate Innovation Programs
RICH has worked with various corporates in scouting for the potential start-ups that can work along with them
Ecosystem
1. ABCs of Medical Devices and In-vitro Diagnostics (IVDs) Commercialisation Journey
An annual flagship program of RICH offering hands-on workshops for aspiring entrepreneurs, researchers, start-ups, MSMEs, SMEs, and industry players. The series focuses on four tracks: intellectual property, regulatory, industry immersion, and clinical exposure. Each track led by experts in the field, providing participants with in-depth knowledge and practical insights into the various stages involved in scaling a proof-of-concept validated idea to a marketable product.









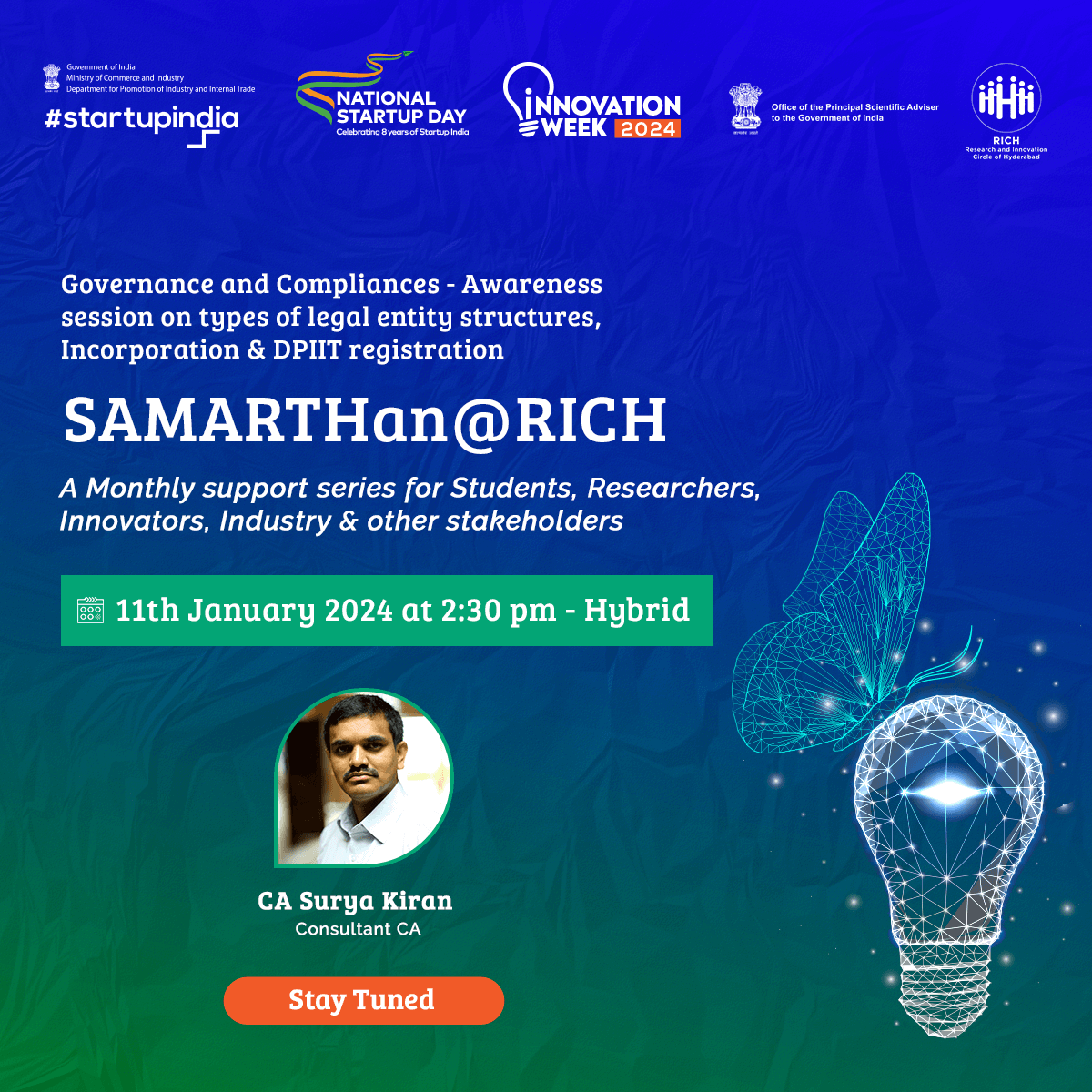
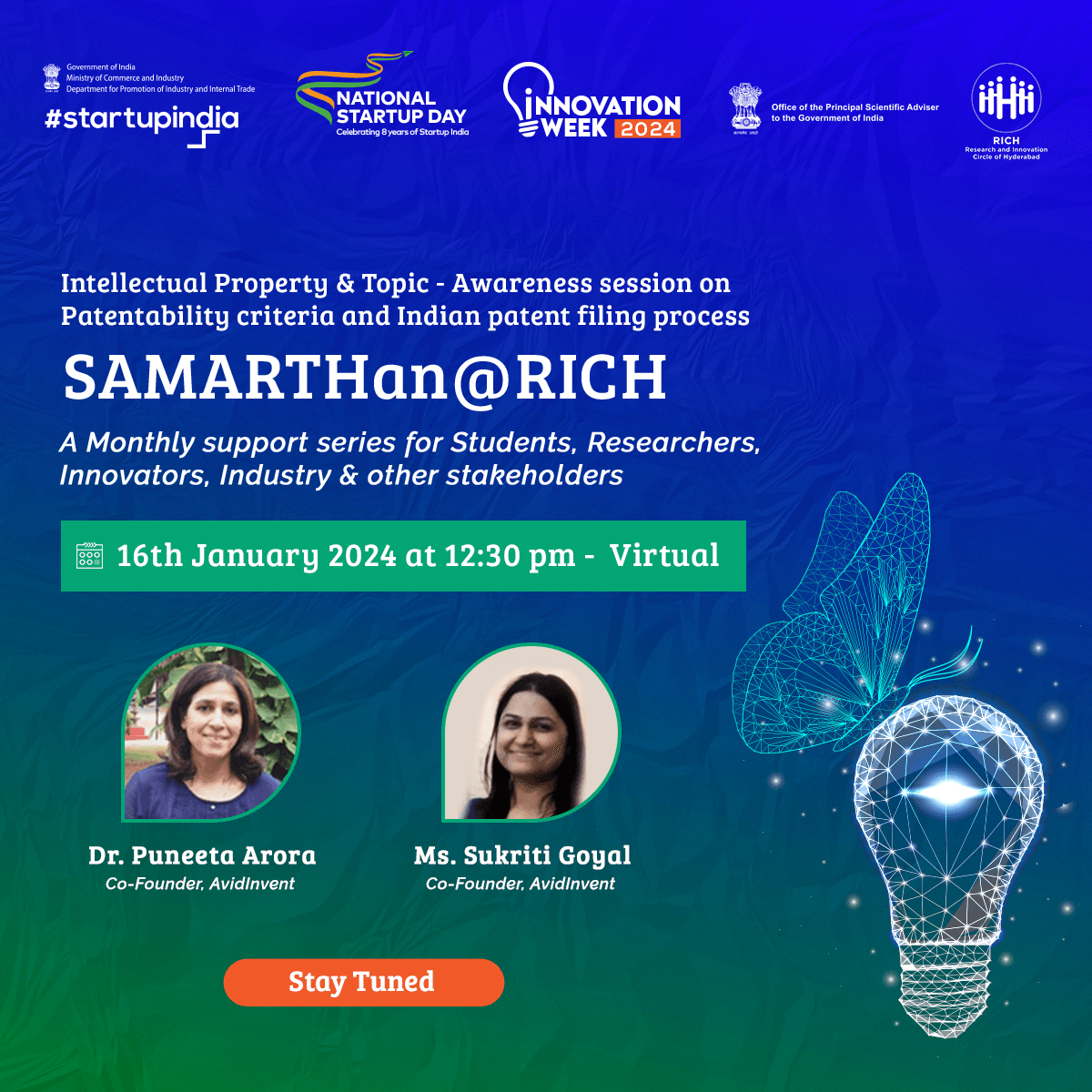
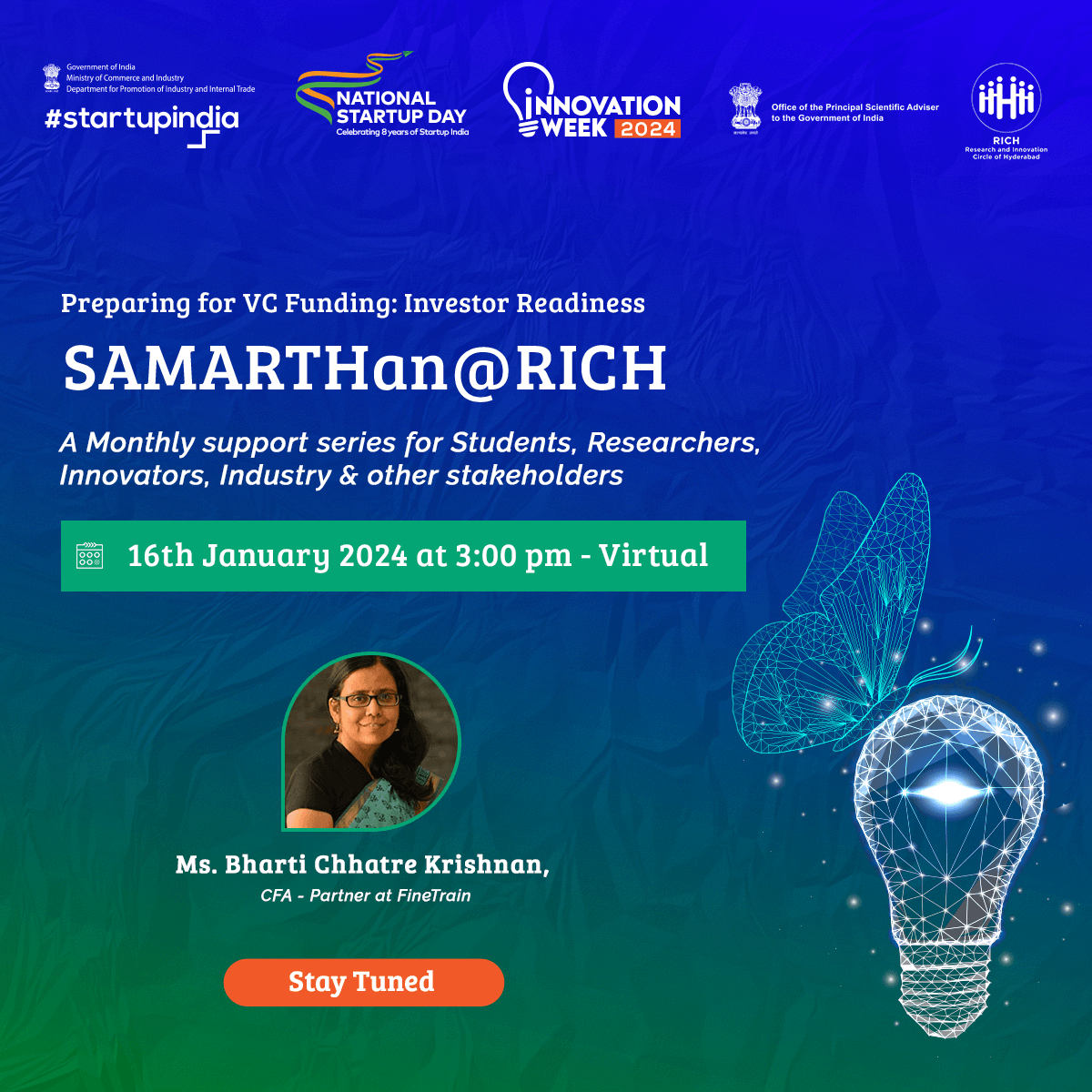
SAMARTHan @ RICH
Designed to address the challenges faced by medical technology (MedTech) innovators and startups as they bring their products to market through its office hours -
SAMARTHan@RICH offers a comprehensive program covering critical areas like:
- Intellectual Property (IP) awareness and application process
- Governance and compliance
- Idea validation from clinicians
- Regulatory roadmap for product development and commercialization
- Conducting clinical validation studies
Projects
1. Women in STEM

The programme provides an unique internship opportunity for under-graduate and post-graduate women students in their final year to spend two (2) to six (6) months in labs of premier R&D institutions or industries.




